Laboratory of Early Mammalian Developmental Biology (LEMDB)
Research in the LEMDB is focussed on understanding the molecular mechanisms that underpin mammalian preimplantation embryo development; specifically the derivation of the first three cell lineages (trophectoderm, primitive endoderm & epiblast) that arise by the time of embryo uterine implantation. We are also investigating the fundamental processes that control the formation of viable mouse oocyte.
Head of the laboratory: prof. Alexander W. Bruce, Ph.D.
Post-doctoral researchers:
Valeriya Zabelina, Ph.D.
Monika Fluks Ph.D.
Ph.D. students:
Rebecca Collier, M.Sc.
Mgr. Martina Stiborová
Mgr. Andrea Hauserová Bc.
*** POTENTIAL Ph.D. POSITIONS AVAILABLE (contact - This email address is being protected from spambots. You need JavaScript enabled to view it.) ***
Masters & Bachelors students (all applications considered):
no current Masters students
Francesca Botnari
Nina Bock
Štěpán Kubart
* ALWAYS OPEN TO SPECULATIVE APPROACHES FOR RESEARCH PROJECTS FROM TALENTED & DEDICATED BACHELORS & MASTERS STUDENTS *
Read all about it!
LATEST LAB NEWS
Molecular mechanisms regulating preimplantation mouse embryo development and cell-fate
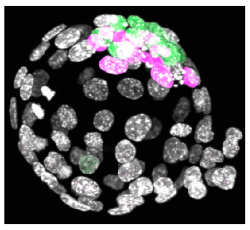
Following oocyte fertilization, the mammalian zygote undergoes a series of prolonged cell cycle divisions that results in the generation of the mature blastocyst consisting of three distinct cell types. The outside surface of the embryo comprises an epithelium of differentiated cells called the trophectoderm (TE – that subsequently develop to form the embryonic component of the placenta - see white nuclei opposite), whilst a second population of differentiated cells, the primitive endoderm (PE – that primarily give rise to cells of the yolksac - magenta stained nuclei), is found inside the embryo in contact with a fluid filled cavity and is distinct from other inner cells, the epiblast (EPI – that are the pluripotent progenitors cells of the foetus proper - green stained nuclei), that are completely buried inside the blastocyst structure.
The differentiated TE and PE cells eventually develop after implantation to support and direct development of the foetus that is itself derived from the EPI population of cells. However, the early mammalian embryo can exhibit extraordinary regulative behaviour and can successfully develop even when cells or added or removed. Despite this developmental flexibility, evidence is emerging that early inter-cell asymmetries are able to bias individual cell-fate in semi-predictable ways suggestive of at least some early patterning in the mammalian embryo.
Consequently, our primary research objectives are to uncover novel molecular mechanisms that impact upon successful blastocyst formation under both unperturbed and regulative conditions. Furthermore, to better understand how the blastocyst’s three constituent lineages are derived from a single totipotent zygote during the considerable spatio-temporal constraints of pre-implantation development and to probe the functional importance of very early inter-cell asymmetries.
We employ classical embryological and molecular biological techniques to probe the mechanisms of action of candidate cell fate influencing genes, identified in genomic scale expression screens of themouse embryo and mouse ES cells. Hence, we anticipate that our multi-disciplined approach, investigating the ‘in vivo’ balance between cell differentiation and retention of pluripotency in early mouse embryogenesis, will not only advance our fundamental knowledge of one of the most enigmatic periods of development but will also provide insight to clinical/ veterinary embryologists and those clinicians working in the field of regenerative stem cell-based medicine.
Regulation of mouse oocyte formation
In female humans and mice the process of oocytogenesis is completed around the time of birth and the prevailing dogma dictates that no further primary oocytes are generated thereafter (in stark contrast to continuous spermatogenesis in males). The process of forming ootids (ootidogenesis) occurs during two rounds of meiosis and is initiated prenatally. However, ootidogenesis is stalled in the prophase stage of meiosis I so that by the time of birth the development of all the primary oocytes within the ovary is halted at this stage. Under the influence of the menstrual cycle hormones (follicle stimulating hormone and luteinizing hormone), meiosis I resumes in a sub-population of primary oocytes (and there is a time window in which sister chromosomes can recombine leading to genetic cross over). Ovulation then takes place and the haploid secondary oocyte plus the first polar body are generated, at which time meiosis II of ootidogenesis is initiated but is then arrested at the metaphase II stage. Upon sperm fertilisation, the secondary oocyte resumes meiosis II and extrudes the second polar body, thus forming the short-lived ootid, that then undergoes further maturation steps as a fertilised ovum, eventually forming a diploid zygote capable of further embryonic development.
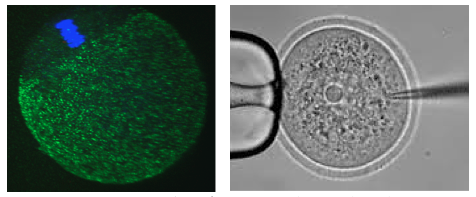
In our laboratory, we are interested in learning more about the molecular processes occurring when diploid primary oocytes are recruited into ootidogenesis and then mature into haploid secondary oocytes capable of being fertilised and supporting the preimplantation stages of embryonic development. We are particularly interested in the mechanisms that ensure the faithful segregation of chromosomes between the maturing oocyte and the extruded polar bodies (during meiosis I and II) and are functionally characterising a number of candidate target genes (using classical mouse genetics and RNAi/ mRNA microinjection and high resolution time-lapse microscopy techniques on oocytes maturing in culture).
Fluks M, Collier R, Walewska A, Bruce AW, Ajduk A. How great thou ART: Biomechanical properties of oocytes and embryos as indicators of quality in assisted reproductive technologies. Front. Cell Dev. Biol. in press (2024). IF2022 = 5.5.
Iyyappan R, Aleshkina D, Ming H, Dvoran M, Kakavand K, Jansova D, Del Llano E, Gahurova L, Bruce AW, Masek T, Pospisek M, Horvat F, Kubelka M, Jiang Z, Susor A. The translational oscillation in oocyte and early embryo development. Nucleic Acids Res. gkad996 (2023). IF2022 = 14.9
Gahurova L, Tomankova J, Cerna P, Bora P, Kubickova M, Virnicchi G , Kovacovicova K, Potesil D, Hruska P, Zdrahal Z, Anger M, Susor A and Bruce AW. Spatial positioning of preimplantation mouse embryo cells is regulated by mTORC1 and m7G-cap dependent translation at the 8- to 16-cell transition. Open Biology 8:230081 (2023). IF2022 = 5.800
Bora P , Gahurova L, , Hauserova A, Stiborova M, Collier R, Potěšil D, Zdráhal Z and Bruce AW. DDX21 is a p38-MAPK sensitive nucleolar protein necessary for mouse preimplantation embryo development and cell-fate specification. Open Biology 7:210092 (2021). IF2021 = 6.411 .
Bora P , Gahurova L, Mašek T, Hauserova A, Potěšil D, Jansova D, Susor A, Zdráhal Z, Ajduk A, Pospíšek M and Bruce AW. p38-MAPK-mediated translation regulation during early blastocyst development is required for primitive endoderm differentiation in mice. Commun. Biol. 4:788 (2021). IF2021 = 6.268
Virnicchi G, Bora P, Gahurova L, Šušor A and Bruce AW. Wwc2 is a novel cell division regulator during preimplantation mouse embryo lineage formation and oogenesis. Front. Cell. Dev. Biol. 8:857. (2020). IF2020 = 6.684
Del Llano E, Masek T, Gahurova L, Pospisek M, Koncicka M, Jindrova A, Jansova D, Iappan R, Roucova K, Bruce AW, Kubelka M and Susor A. Age-related differences in the translational landscape of mammalian oocytes. Aging Cell e13231 (2020). IF2020 = 9.304
Masek T, Del Llano E, Gahurova L, Kubelka M, Susor A, Roucova K, Lin C-J, Bruce AW and Pospisek M. Identifying the translatome of mouse NEBD-stage oocytes via SSP-profiling; a novel polysome fractionation method. Int. J. Mol. Sci. 21(4) 1254 (2020). IF2020 = 5.923
Bora P, Thamodaran V, Šušor A and Bruce AW. p38-mitogen activated kinases mediate a developmental regulatory response to amino acid depletion and associated oxidative stress in mouse blastocyst embryos. Front. Cell. Dev. Biol. 7:276 (2019). IF2019 = 5.186
Koncicka M, Tetkova A, Jansova D, Del Llano E, Gahurova L, Kracmarova J, Prokesova S, Masek T, Pospisek M, Bruce AW, Kubelka M and Susor A. Increased expression of maturation Promoting Factor components speeds up meiosis in oocytes from aged females. Int. J. Mol. Sci. 19(9), 2841 (2018). IF2018 = 4.183
Mihajlović AI and Bruce AW. The first cell-fate decision of mouse preimplantation embryo development: integrating cell position and polarity. Open Biology. 7(11) /rsob.170210 (2017). IF2017 = 3.286
Koštál V, Štětina T, Poupardin R, Korbelová J, Bruce AW. Conceptual framework of the eco-physiological phases of insect diapause development justified by transcriptomics profiling. Proc. Natl. Acad. Sci. USA. 114(32), 8532-8537 (2017). IF2017 = 9.504
Thamodaran V and Bruce AW. p38 (Mapk14/11) occupies a regulatory node governing entry into primitive endoderm differentiation during preimplantation mouse embryo development. Open Biology. 6(9) /rsob.160190 (2016). IF2016 = 3.481
Mihajlović AI and Bruce AW. Rho-associated protein kinase regulates subcellular localisation of Angiomotin and Hippo-signalling during preimplantation mouse embryo development. Reprod. Biomed. Online. 33(3), 381-390 (2016). IF2016 = 3.249
Mihajlovic AI, Thamodaran V and Bruce AW. The first two cell-fate decisions of preimplantation mouse embryo development are not functionally independent. Scientific Reports 13(5): 15034 (2015). IF2015 = 5.228
Funding
2020-2022: Czech Science Foundation (CSF): 21-03305S: ‘Characterisation of Hippo-signalling related genes during mouse oocyte maturation, acentrosomal cell division & blastocyst cell lineage allocation.’ Duration: 3 years. Finance: 8,672,000 CZK.
2018-2020: Czech Science Foundation (CSF): 18-02891S: ‘Regulating the balance between differentiation and pluripotency; molecular characterisation of p38-MAPK function in mouse blastocyst ICM maturation.’ Duration: 3 years. Finance: 6,247,000 CZK.
2017-2021: Marie Curie Career Individual Fellowship Grant (awarded jointly with a post-doctoral researcher, Lenka Gahurová Ph.D., who is hosted in my group; H2020): OOCSOCS: ‘Socs3 gene in oocyte maturation and fertilsation – a novel link between inflammation and infertility’. Duration: 3 years. Finance: 142, 720 EUR - reinstated after maternity leave (LG).
2013-2016: Czech Science Foundation (CSF): 13-03295S: ‘Functional characterisation of identified novel candidate cell-fate influencing genes during pre-implantation mouse development’. Duration: 4 years. Finance: 6,889,000 CZK.
2011-2015: Marie Curie Career Integration Grant (within the 7th European Community Framework Programme): IDNOVCELFAT2011: ‘Identification and characterization of novel cell-fate influencing genes in pre-implantation mouse development’. Duration: 4 years. Finance: 100,000 EUR.
The Visegrád Group Society for Developmental Biology (V4SDB) 
The LEMDB is an active supporter of the Visegád Group Society for Developmental Biology (V4SDB) with Alexander W. Bruce serving as a founder member of organising committee. The V4SDB is committed to supporting excellence in the field of developmental biology research throughout the Czech Republic, Poland, Hungary and Slovakia and is actively engaged in outreach and other collaborative efforts. See the V4SDB homepage http://v4sdb.org/index
The Inaugural Meeting of the V4SDB was held in the Czech city of Brno from 7-9th September 2018. You can find more details about the society and the Brno meeting on conference webpage (https://webcentrum.muni.cz/visegrad2018) or the V4SDB Facebook page: www.facebook.com/V4SDB
The Second V4SDB Meeting (COVID-19 delayed) took place in Szeged (Hungary) from 2nd-5th September 2021 -You can find more details about the Szeged meeting on the conference webpage (https://www.v4sdbszeged.com/) or the V4SDB Facebook page: www.facebook.com/V4SDB
The Third V4SDB Meeting was held in Warsaw (Poland) from 8th-10th September 2023 - You can find more details about the Szeged meeting on the conference webpage (https://v4sdb2023.pl/welcome_message) or the V4SDB Facebook page: www.facebook.com/V4SDB
The Fourth V4SDB Meeting is due to be held in Warsaw (Poland) in September 2025 - more details to follow: https://www.v4sdb.org/2023-v4sdb-meeting-warsaw-1-1
Laboratory alumni (Where are they now?)
Former post-doctoral researchers
Lenka Gahurová (2023 – former Marie Curie Individual Grant Fellowship holder): Faculty member and Assistant Professor at the University of South Bohemia in České Budějovice (Department of Molecular Biology & Genetics – Laboratory of Mammalian Epigenetics and Bioinformatics), Czech Republic.
Ender Yalcinkaya (2013): Eurofertil In Vitro Fertilization Center, Kocaeli University IVF unit, Embryology Laboratory, Istanbul, Turkey.
Former Ph.D. students:
Pablo Bora (2021): University of Massachusetts Medical School - laboratory of prof. Oliver J. Rando - https://www.umassmed.edu/randolab/
Giorgio Virnicchi (2021): PLAISANT, Rome, Italy - Transgenic Mouse Generation outsourcing company - http://www.plaisant.eu/
Aleksandar Mihajlović (2017): Centre de Recherche du Centre Hospitalier de l’Université de Montréal, Montréal, Canada (recipient of FRQS post-doctoral fellowship - 2019) - https://www.fitzharrislab.com/
Vasanth Thamodaran (2016): Tata Institute for Genetics and Society - TIGS, Bengaluru, India (recipient of INSPIRE Faculty Fellowship/ Early Career - Indian National Academy of Sciences - 2020) - https://tigs.res.in/people/scientists/vasanth-thamodaran/
Former Master's students:
Valeriy Kutsnya (2023): Ph.D. student: Research Centre at the University of Montreal Hospital CRCHUM (Canada; Prof. Greg Fitzharris, Ph.D.).
Pavlína Černá (2021): Pronatal Repro Human IVF clinic in České Budějovice (Budweis), Czech Republic.
Miriama Pekl’anská (2021): Ph.D. student: Institute of Parasitology, (AV ČR - Budweis); Laboratory of Molecular Biology of Ticks (RNDr. Radek Šíma, Ph.D.)
Michaela Kubíčková (2018): Czech TEAM Olympic Beach Volleyball Athlete.
Study at the Faculty
-
Grading system
-
Study and Examination Code
Branišovská 1645/31a, 370 05 České Budějovice Tel. 387 776 201 | This email address is being protected from spambots. You need JavaScript enabled to view it.
Branišovská 1645/31a, 370 05 České Budějovice Tel. 387 776 201 | This email address is being protected from spambots. You need JavaScript enabled to view it.
© 2024 University of South Bohemia
Cookies
1
0



